
About Us Cibis Park
Bisoprolol was the first beta-blocker shown to have beneficial effects on outcomes in the Cardiac Insufficiency Bisoprolol Study II (CIBIS-II) ( CIBIS-II Investigators and Committees 1999 ). The aim of this article is to review its main pharmacological characteristics with respect to its use in the patients with HF. Go to:

CIBIS Nine at CIBIS Park Cibis Park
Bisoprolol reduced all cause mortality by 32% (11.8% vs 17.3%, p=0.002). Sudden death was reduced by 45% (3.6% vs 6.4%, p=0.001).. CIBIS-II was the first randomized controlled trial with sufficient power to address all cause mortality as a primary objective. The mortality benefit of low-dose beta blockade in congestive heart failure is.

Pin on Smart Buildings
Findings: CIBIS-II was stopped early, after the second interim analysis, because bisoprolol showed a significant mortality benefit.. (156 [11.8%] vs 228 [17.3%] deaths with a hazard ratio of 0..

Cibis Park, Tempat Rekreasi Gratis Warga Jakarta Rumika's Journey
The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial Summary Background In patients with heart failure, β-blockade has improved morbidity and left-ventricular function, but the impact on survival is uncertain.

Gallery of Cilandak Bisnis Square (CIBIS) Masterplanning Project
The investigators randomized 1010 patients with mild to moderate heart failure, and an ejection fraction <35%, to open-label monotherapy with either bisoprolol (target dose 10 mg/day) or enalapril (target dose 10 mg twice daily) for 6 months followed by uptitration of the second drug for 6 to 24 months.

Cibis Tower 9
The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial 1999 Jan 2;353 (9146):9-13. PMID: 10023943 In patients with heart failure, beta-blockade has improved morbidity and left-ventricular function, but the impact on survival is uncertain.

CIBIS Nine at CIBIS Park Cibis Park
Findings CIBIS-II was stopped early, after the second interim analysis, because bisoprolol showed a significant mortality benefit. All-cause mortality was significantly lower with bisoprolol than on placebo (156 [11·8%] vs228 [17·3%] deaths with a hazard ratio of 0·66 (95% CI 0·54-0·81, p<0·0001). There were significantly fewer
EightID Cibis Park
1. CIBIS-II Investigators and Committees The Cardiac Insufficiency Bisoprolol II (CIBIS-II): a randomised trial. Lancet. 1999; 353: 9-13 View in Article Scopus (4219) PubMed Summary

CIBIS Tower Nine
The 1999 Cardiac Insufficiency Bisoprolol Study II (CIBIS-II) demonstrated that in patients with HFrEF (defined as LVEF ≤35%) and NYHA class III-IV symptoms, the beta blocker bisoprolol results in a 34% relative risk reduction in all-cause mortality (5% absolute reduction) when added to standard therapy including ACE inhibitors and diuretics.

CIBIS Nine at CIBIS Park Construction Plus Asia
Comparison of CIBIS meta-analysis with more recently published trials. The results of the CIBIS and CIBIS II meta-analysis were evaluated and combined with the results for total death of comparable older 5 and more recently published trials in which other β-blockers were tested 6, 7, 8 in a further meta-analysis (Figure 7).

Broadway Malyan Appointment adds further towers to CIBIS role
Bisoprolol was evaluated in the CIBIS-II trial, leading to all-cause mortality of 8.8% versus 13.2% in the placebo group (P < 0.0001).. (P < 0.001) and 2.8% (P < 0.001), respectively. 5 The total daily strength of the combination product used in the trial offered bioavailability similar to 320 mg valsartan. Although this is the desired.

RetailLink Cibis Park
CIBIS II trial The CIBIS II trial was based on the first CIBIS study, which showed a non-significant 20% reduction in mortality but a significant reduc-tion in hospital admissions as a result of wors-ening heart failure.67CIBIS II was a much larger trial,comprising a total of 2647 patients. It was the first of the very large trials with suf-

About Us Cibis Park
A recently derived 8-item version of the SIB—the SIB-8—which takes about 3 minutes to administer, may represent a more convenient tool for use in clinical practice. The current analyses further explored the SIB-8 scale with respect to its validity and sensitivity. Methods

CIBIS Nine, Jakarta
The CIBIS II trial was based on the first CIBIS study, which showed a non-significant 20% reduction in mortality but a significant reduction in hospital admissions as a result of worsening heart failure. 6 7 CIBIS II was a much larger trial, comprising a total of 2647 patients.
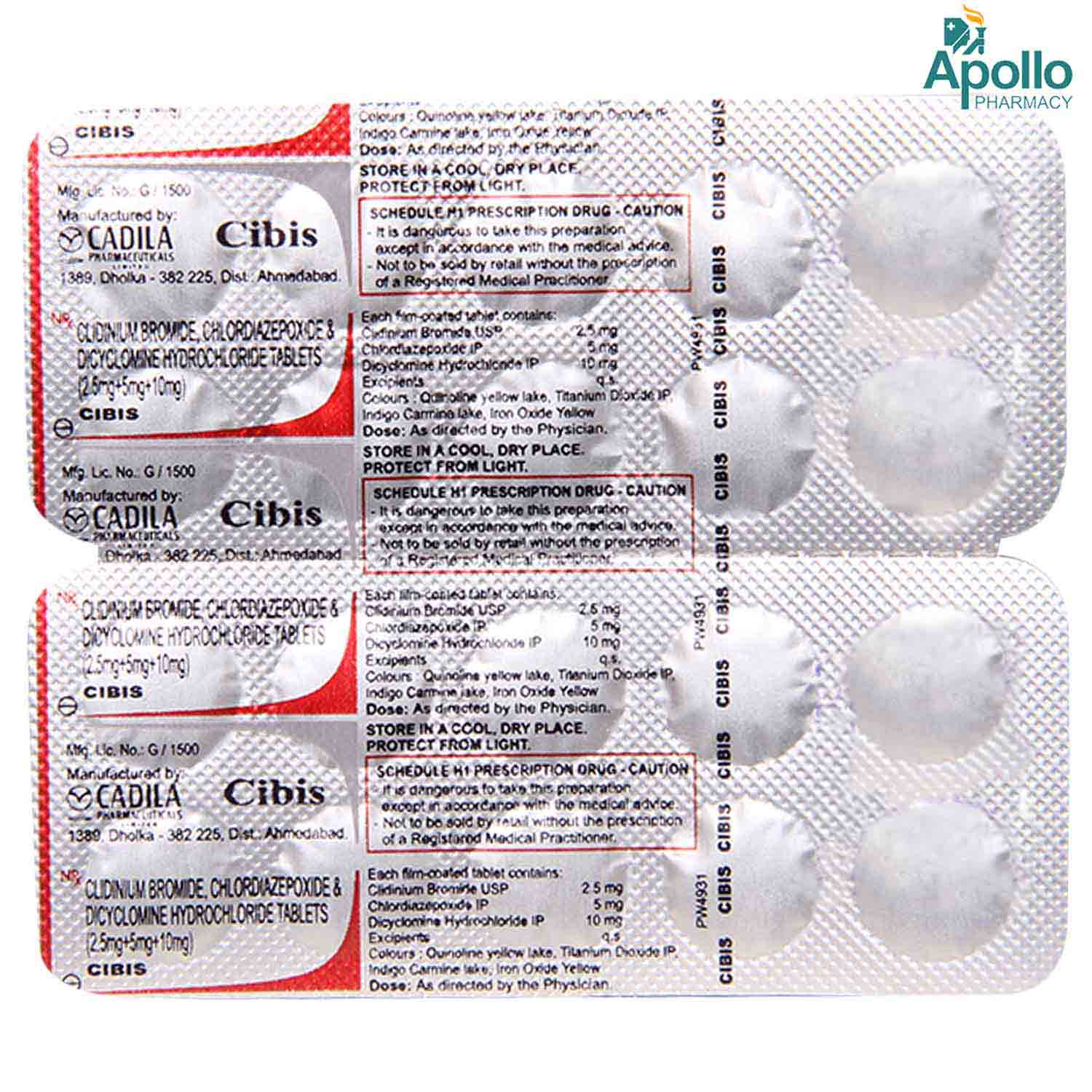
CIBIS TABLET Price, Uses, Side Effects, Composition Apollo Pharmacy
Bisoprolol fumarate is a highly selective beta-1 receptor blocker. Bisoprolol has been extensively studied in three large mortality trials in stable chronic heart failure (CHF) patients. The CIBIS trial enrolled 641 patients and demonstrated the good tolerability of bisoprolol in a large CHF population, without evidence for any harmful effect.

CIBIS Nine at CIBIS Park Cibis Park
In CIBIS, idiopathic dilated cardiomyopathy was diagnosed when no known cause of cardiomyopathy could be found. Patients were classified as having ischaemia when there was a typical history of coronary artery disease, a history of myocardial infarction, or the presence of a coronary stenosis greater than 70% shown by coronary angiography.